|
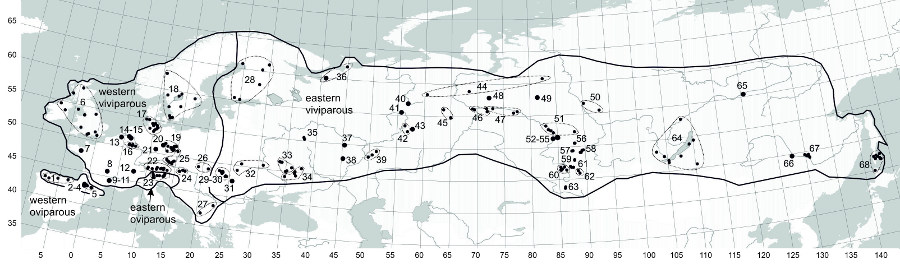
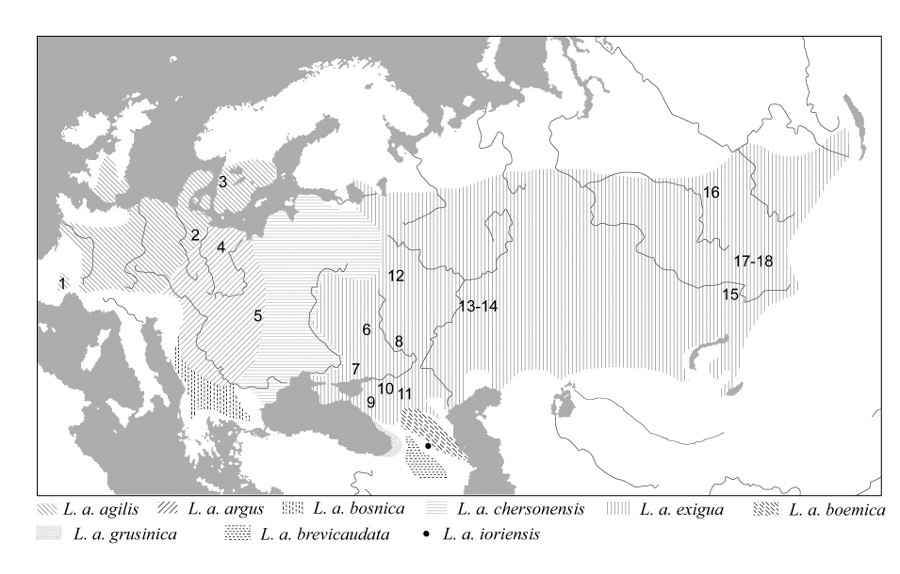
Statement of research interestsVariation in adult body size, sexual size dimorphism, and female reproductive output in lacertid lizardsDuring last three years, my research activity has concentrated on the project “Patterns and determinants of geographic variation in adult body size and sexual size dimorphism in two widespread lizard species” supported by the Deutsche Forschungsgemeinschaft (grant RO 4168/1–1). Intraspecific variation is an important issue because it links macroevolutionary patterns to microevolutionary processes which lead to the phenotypic diversity we wish to understand. Wide-ranging species present promising models for simultaneously evaluating the role of different factors shaping the phenotypic diversity, because the variation of target traits can be documented from numerous localities exhibiting diverse combinations of putative predictors (Roitberg et al. 2013). However, comprehensive range-wide studies of geographic variation in widespread species are rare, even for fundamentally important traits such as adult body size, offspring size and fecundity. In cooperation with many herpetologists from Western and Eastern Europe, Ukraine, and Russia I am studying macrogeographic and regional patterns of variation in body size, sexual size dimorphism, and female reproductive output in the two most widespread terrestrial reptiles in the world, the lacertid lizards Lacerta agilis and Zootoca vivipara. Our data set currently encompasses over 20,000 individual values of several body-size and reproductive traits (Table 1) from more than 100 local and regional samples across the distributional ranges of the study species ( Fig. 1; Fig. 2 ). Table 1. Summary of the current data set. This table includes original data and individual-based data extracted from published figures. Published mean values used in our analyses (e.g., Roitberg et al. 2013) are not considered here.
Fig. 1. Geographic ranges of different clades of Zootoca vivipara and our study samples for body length data. Our study samples for reproductive data are shown in Roitberg et al. (2013, Fig. 1).
Fig. 2. Geographic disribution of different subspecies of Lacerta agilis (after Roitberg 2007) and our study samples for reproductive data. Our study samples for body length data are much more numerous than those for reproductive traits. These data will be summarized later because the expectations for additional material are high To address a series of evolutionary hypotheses we investigate relationships of the variation of the studied life-history traits with environment (climate), with ancestry (intraspecific evolutionary lineage), and among each other (Roitberg 2007; Roitberg et al. 2012, 2013, Roitberg et al., in prep.). An important point of our approach is a careful consideration of potential confounding factors. The effects of several confounding factors, including the type of material (live vs. preserved specimens)(i), intra- and inter-observer differences in defining adult samples (ii) and measuring body length (iii) were examined. The issue (iii) was studied in greatest detail. The data collected till mid-2011 are summarized in Roitberg et al. (2011). Since then I have proceeded to collect such data, and a total of 16 herpetologists (including nearly all major contributors for both species) have been tested. For the vast majority of the tested observers, mean bias relative to my own measurements located within -1 and + 1 mm (Roitberg et al., 2011 and unpubl. data). In overall, ca. 700 repeated measurements have been done. Our study of intraspecific variation in adult body size, sexual size dimorphism, and female reproductive output in Lacerta agilis and Zootoca vivipara is in progress, and colleagues who possess appropriate data are welcome to cooperate with us. Investigations of life-history variation in lizards using skeletochronologyDuring last decades, lizards have been among the model animal groups for studying factors which determine the variation of life-history and demography traits within and among species. These studies have developed an advanced theoretical and methodological framework, but the weak point is that they cover only a small set of populations and taxa. The problem is that both mark-recapture and experimental studies - the main tool to obtain data on growth and longevity in wild animals - are very time-consuming. A promising alternative tool to get such data is the recording structures analysis (Mina & Klevezal, 1970; Klevezal, 1988), also known as skeletochronology (Castanet et al. 1977). This method based on counting and measuring growth layers in bone or other hard tissues provides not only an accurate age determination in reptiles and amphibians, but also a quantitative estimation of the pattern of bone growth (Smirina, 1969, 1994; Castanet, 1970, 1994; Castanet & Smirina, 1990). Moreover, individual trajectories of body growth can be back-calculated from the bone growth marks (Smirina, 1983; Marunouchi et al., 2000, etc.). In cooperation with E. M. Smirina (N. K. Koltsov Institute of Developmental Biology, research team of G. A. Klevezal) I have studied altitudinal variation and sex differences for age structure, asymptotic body size and growth pattern in two related lizard species (Lacerta agilis boemica and L. strigata) from the Caucasus. The data were obtained from preserved specimens, using skeletochronology and back-calculation methods. As nobody before had applied the back-calculation technique to squamate reptiles, methodological problems which can arise by inferences about body growth from skeletochronological data have been another important target of our study (Roitberg & Smirina, 1995, 2005, 2006a). Trends and Constraints in Morphological VariationMy previous research involved various aspects of intraspecific morphological variability in lacertid lizards. Reptilian pholidosis is an excellent model for studying general regularities in variation and evolution of morphological structures. This is particularly true for lacertid lizards whose scutellation consists of well-defined series providing plenty of precisely countable characters. Although these characters are widely used in intraspecific taxonomy as well as in microevolutionary studies the morphological content of the variation usually remains out of special analysis. Studying variation of pholidotic traits in several species of the genera Lacerta and Darevskia, I addressed various topics like the intrinsic trends of variability due to developmental constraints ( Roitberg, 1991, 1999b; Roitberg & Rostova, 1999 and in prep.), morphological aspects of speciation ( Roitberg, 1999a ), differences in the level of phenetic differentiation in related sympatric species ( Roitberg, 1994 ). |